Neon, a noble gas, exhibits unique properties stemming directly from its atomic arrangement. This article delves into the intricacies of the atomic structure of neon, a subject often explored through the lens of quantum mechanics. Specifically, understanding neon’s electron configuration, which is crucial for its inertness, requires knowledge of the Pauli Exclusion Principle. Scientific investigations at CERN, although not exclusively focused on neon, contribute significantly to the broader understanding of atomic nuclei and electron behaviors. These elements contribute to an atomic model, particularly the Bohr model, and are often used in educational settings to conceptualize the nature of atomic element.
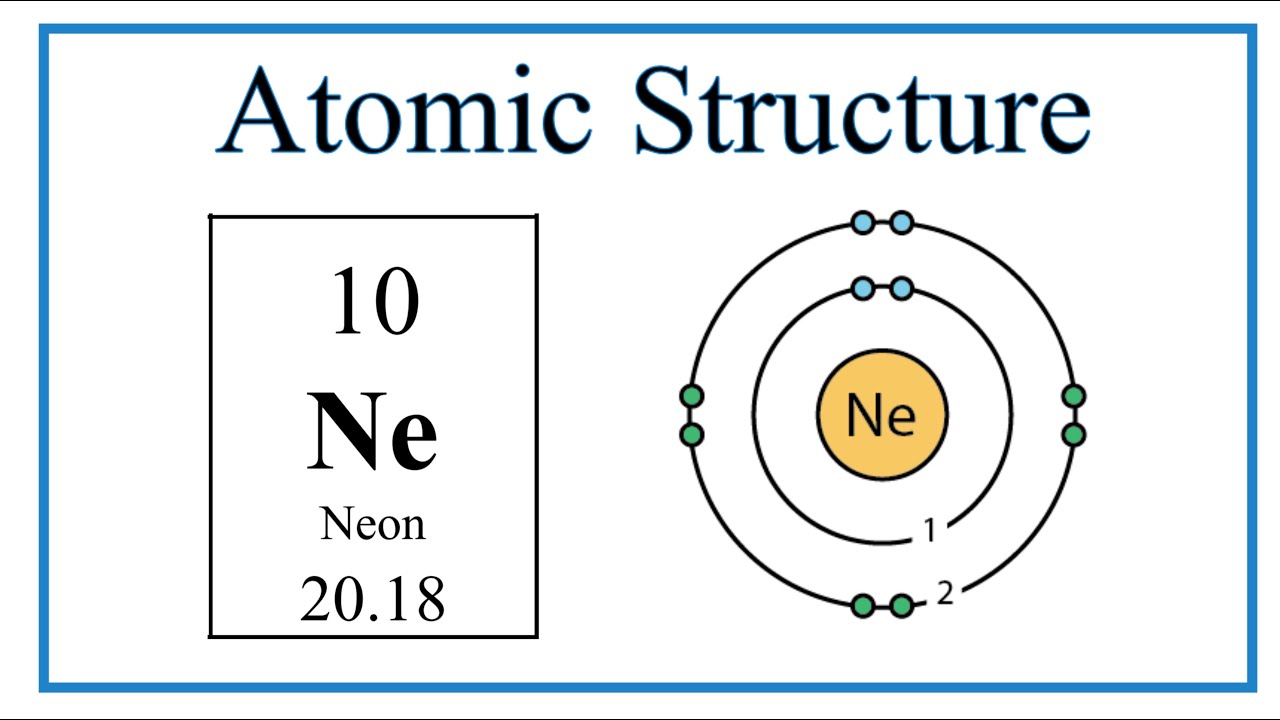
Image taken from the YouTube channel Wayne Breslyn (Dr. B.) , from the video titled Atomic Structure (Bohr Model) for the Neon (Ne) Atom .
Neon, symbolized as Ne on the periodic table, is instantly recognizable for its vibrant glow in signs and lighting. This element, however, is more than just a source of vivid color. It holds a unique position in the world of chemistry as a Noble Gas, possessing an unparalleled level of stability that sets it apart from many other elements.
This remarkable stability originates from its very atomic structure. Its fundamental components and electron configuration dictate its behavior and properties. This article embarks on an exploration into the atomic structure of Neon, offering a detailed explanation of how its composition dictates its unique characteristics.
A Familiar Glow: Neon’s Everyday Applications
Neon’s most visible application lies in the ubiquitous "neon signs". These signs, created by passing an electrical current through neon gas, produce a brilliant, reddish-orange light.
However, the color can be altered by mixing neon with other gases. This produces a range of hues beyond its signature orange.
Beyond signage, neon finds applications in:
- High-voltage indicators
- Vacuum tubes
- Cryogenics (due to its ability to remain a gas at extremely low temperatures)
The Enigmatic Noble Gas: Stability Defined
As a Noble Gas, Neon belongs to a select group of elements renowned for their inertness or lack of chemical reactivity. This exceptional stability stems from their complete outermost electron shells.
This means they have little to no tendency to form chemical bonds with other elements. Neon’s inert nature makes it invaluable in applications where a non-reactive atmosphere is required.
Understanding the "why" behind Neon’s inertness requires a journey into its atomic structure. It requires an examination of its protons, neutrons, and, most importantly, the arrangement of its electrons.
As we begin to dissect the atomic structure of Neon and its unique stability, it’s essential to understand the fundamental components that make up every atom. Like tiny solar systems, atoms are composed of a central nucleus orbited by electrons. These subatomic particles, their properties, and their interactions are crucial in defining the characteristics of an element.
The Fundamental Building Blocks: Subatomic Particles
At the heart of every atom, including Neon, lies a dense nucleus, the atom’s central core. Surrounding this nucleus are electrons, negatively charged particles that exist in specific energy levels. Understanding the composition and behavior of these subatomic particles is key to unlocking the secrets of Neon’s stability and inertness.
The Nucleus: The Heart of the Atom
The nucleus, a tiny region at the center of the atom, is home to two types of particles: protons and neutrons.
Protons carry a positive charge, while neutrons are electrically neutral. It’s the interplay of these particles that determines the nucleus’s mass and charge, and consequently, the identity of the element.
Composition: Protons and Neutrons
The nucleus is not a homogenous blob; rather it’s a tightly packed collection of protons and neutrons.
The number of protons defines the element; for example, every atom with 10 protons is, by definition, Neon.
Neutrons, while not contributing to the element’s charge, play a crucial role in stabilizing the nucleus.
Relevance to Mass Number
The mass number of an atom is the total number of protons and neutrons in its nucleus.
This number is a crucial identifier, particularly when considering isotopes, which we’ll discuss next.
The mass number gives us a sense of the atom’s overall weight and how it interacts with other atoms and molecules.
Isotopes of Neon and Their Implications
Not all Neon atoms are created equal.
Some Neon atoms have the same number of protons (10) but a different number of neutrons. These are known as isotopes.
Neon has three stable isotopes: Neon-20 (10 protons, 10 neutrons), Neon-21 (10 protons, 11 neutrons), and Neon-22 (10 protons, 12 neutrons).
The existence of isotopes explains why the atomic mass of Neon, as listed on the periodic table, is not a whole number, but rather a weighted average of the masses of its isotopes.
While isotopes of Neon exhibit virtually identical chemical behavior, their differing masses can lead to subtle variations in physical properties, such as rates of diffusion or reaction.
Electrons: Orbiting the Nucleus
Orbiting the nucleus are the electrons, negatively charged particles that are far smaller and lighter than protons and neutrons.
It is the arrangement and behavior of these electrons that dictates how Neon interacts (or, more accurately, doesn’t interact) with other elements.
Location: Energy Levels
Electrons do not orbit the nucleus in random paths. Instead, they occupy specific energy levels, also known as electron shells.
These energy levels are quantized, meaning that electrons can only exist at certain discrete energy values.
Electrons closest to the nucleus have the lowest energy, while those farther away have higher energy.
Neon has two energy levels: the first, closest to the nucleus, can hold a maximum of two electrons, and the second can hold up to eight.
Their Role: Dictating Chemical Behavior
The electrons in the outermost energy level, known as the valence shell, are responsible for an atom’s chemical behavior.
It is the configuration of these valence electrons that determines whether an atom will readily form chemical bonds with other atoms.
In Neon’s case, its valence shell is completely full with eight electrons, making it exceptionally stable and unreactive.
Understanding the behavior of electrons requires delving into the realm of quantum mechanics.
Classical physics fails to accurately describe the behavior of these tiny particles.
Quantum mechanics tells us that electrons do not have definite positions, but rather exist in a probability distribution around the nucleus.
These probability distributions are described by atomic orbitals, which have specific shapes and energies.
A deeper understanding of quantum mechanics is essential for a complete picture of electron behavior, but for the purpose of this explanation, we can appreciate that the filling of these orbitals dictates an atom’s stability and reactivity.
Decoding the Atomic Number and Electron Configuration
Having explored the fundamental particles that constitute the atom, we now turn our attention to how these components organize themselves within Neon, and how this arrangement dictates its unique properties. Understanding the atomic number and electron configuration is paramount to grasping Neon’s inert nature.
Understanding Neon’s Atomic Number
The atomic number is more than just a sequential identifier; it’s the fingerprint of an element, the key to its identity. For Neon, this number holds profound significance.
Defining Neon’s Identity on the Periodic Table
The atomic number of an element unequivocally defines its position on the Periodic Table. Neon, with an atomic number of 10, occupies the tenth slot, nestled among the noble gases. This placement isn’t arbitrary; it reflects the underlying electronic structure that imparts Neon its characteristic inertness. The Periodic Table, in essence, is organized according to these atomic numbers, showcasing recurring patterns in elemental properties.
Relation to Subatomic Particles
The atomic number directly corresponds to the number of protons present in the nucleus of an atom. In the case of a neutral Neon atom, this number is also equal to the number of electrons orbiting the nucleus. This balance of positive (protons) and negative (electrons) charges ensures electrical neutrality. Therefore, knowing Neon’s atomic number instantly reveals the composition of its fundamental charged particles, laying the groundwork for understanding its electron configuration.
Electron Configuration: Mapping the Electron Arrangement
The electron configuration describes how electrons are distributed within the various energy levels and orbitals of an atom. It’s a detailed map of electron occupancy, vital for predicting an element’s chemical behavior.
Detailed Explanation of Neon’s Electron Arrangement
Neon’s electron configuration is 1s²2s²2p⁶. This notation reveals that Neon has two electrons in its innermost energy level (1s orbital), followed by two electrons in the 2s orbital and six electrons in the 2p orbitals of its second energy level.
This arrangement completes the second energy level, fulfilling the octet rule. This specific configuration is the key to Neon’s stability and lack of reactivity.
Visual Representation
Visualizing Neon’s electron configuration can enhance understanding. A diagram depicting the nucleus surrounded by two electron shells, with the first shell containing two electrons and the second containing eight, provides a clear representation of this stable arrangement.
Such visual aids are invaluable for grasping abstract concepts like electron orbitals and energy levels.
The Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer electron shell containing eight electrons. Neon already possesses this stable configuration, with eight electrons in its valence shell (the outermost electron shell). This fulfills the octet rule without the need to form chemical bonds. As a result, Neon is exceptionally stable and chemically inert, rarely participating in chemical reactions under normal conditions.
The Noble Nature of Neon: Explaining Inertness
Having meticulously mapped Neon’s atomic number and the arrangement of its electrons, we can now fully appreciate the reason behind its remarkable inertness. Neon, unlike many other elements, exhibits an almost complete lack of chemical reactivity. This characteristic defines its classification as a Noble Gas, a group renowned for their reluctance to form chemical bonds.
The Electron Configuration Connection
The key to understanding Neon’s inertness lies within its electron configuration. The specific arrangement of electrons in Neon’s atom directly dictates its chemical behavior.
Unlike elements with partially filled electron shells that actively seek to gain, lose, or share electrons to achieve stability, Neon possesses a particularly stable electron arrangement. This stability is not accidental; it’s a fundamental aspect of Neon’s atomic structure.
The Full Valence Shell and the Octet Rule
The concept of a full valence shell is crucial to explaining Neon’s stability. The valence shell is the outermost electron shell of an atom. Neon’s valence shell contains eight electrons. This completely filled outer shell satisfies the octet rule.
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell containing eight electrons. Because Neon already possesses this optimal configuration, it has no inherent drive to participate in chemical reactions. It’s already stable and content.
Absence of Chemical Bond Formation
The fulfilled octet rule explains why Neon does not readily form chemical bonds with other atoms. Elements form chemical bonds to achieve stability by attaining a full valence shell.
Neon already has a full valence shell, and doesn’t need to gain, lose, or share electrons. As a result, there is no driving force for Neon atoms to interact and create compounds.
This lack of chemical reactivity is what makes Neon so useful in applications where inertness is required, such as in lighting and cooling applications.
Because Neon doesn’t need or want to react with anything, we can rely on its stability and inertness for these applications.
Applications and Importance of Understanding Neon’s Atomic Structure
Having explored the intricacies of Neon’s atomic makeup, from its nucleus to the arrangement of its electrons, we arrive at a pivotal question: Why does this knowledge matter? Understanding Neon’s atomic structure isn’t merely an academic exercise; it unlocks the door to predicting and harnessing its unique properties for a variety of applications.
Predicting Behavior Through Atomic Insight
The true power of understanding atomic structure lies in its predictive capabilities. By knowing Neon’s electron configuration and its adherence to the octet rule, we can confidently assert its inertness.
This understanding allows us to anticipate how Neon will interact (or, more accurately, not interact) with other substances under various conditions. This predictive capability is paramount in scientific research and technological development.
Neon’s Diverse Applications
Neon’s inertness and characteristic emission of light when excited have led to its widespread use in several key areas.
Illumination: The Iconic Neon Signs
Perhaps the most recognizable application of Neon is in the creation of vibrant, eye-catching signs. When an electric current is passed through Neon gas, it emits a distinctive reddish-orange light.
By blending Neon with other gases, different colors can be produced, resulting in the diverse palette of colors seen in neon signs.
Cryogenics: Utilizing Extreme Cold
Beyond its luminous applications, Neon also plays a crucial role in cryogenics – the study and production of extremely low temperatures.
Liquid Neon, with its low boiling point, is used as a refrigerant in specialized applications where other refrigerants, like liquid helium or nitrogen, are not suitable.
High-Voltage Indicators and Lightning Arrestors
Neon is used in high-voltage indicators and lightning arrestors. These applications capitalize on neon’s electrical properties.
Neon’s Place on the Periodic Table: A Noble Legacy
Neon’s position in the periodic table as a Noble Gas underscores its unique properties and significance. As a member of Group 18, it shares characteristics with other inert gases like Helium, Argon, Krypton, and Xenon.
These elements are all characterized by their stable electron configurations and lack of chemical reactivity. Neon’s presence in this group reinforces the periodic trends and provides valuable insights into the relationships between elements and their properties.
Understanding Neon’s Atomic Structure: FAQs
Here are some frequently asked questions to help you better understand the atomic structure of neon and its properties.
What makes neon so stable?
Neon’s stability comes from its full outer electron shell. It has 8 valence electrons, fulfilling the octet rule. This complete outer shell configuration makes the atomic structure of neon very stable and unreactive, which explains why it is a noble gas.
How many protons, neutrons, and electrons does neon have?
A typical neon atom has 10 protons, 10 neutrons, and 10 electrons. The number of protons (10) defines neon’s atomic number. This specific composition of subatomic particles determines the atomic structure of neon.
What does the electron configuration of neon look like?
Neon’s electron configuration is 1s² 2s² 2p⁶. This means it has 2 electrons in its innermost shell (1s) and 8 electrons in its outermost shell (2s and 2p). This configuration is a key aspect of the atomic structure of neon and contributes to its inert nature.
Why does neon glow when electricity passes through it?
When electricity passes through neon gas, the neon atoms become excited. When these excited electrons return to their ground state, they release energy in the form of light, creating the characteristic orange-red glow. This phenomenon is directly related to the atomic structure of neon and the energy levels of its electrons.
So there you have it – a peek inside the atomic structure of neon! Hopefully, this article shed some light on why this element behaves the way it does. Keep exploring the fascinating world of chemistry and see you in the next one!