Understanding the carbon monoxide Lewis structure requires analyzing the electronic configuration of both carbon and oxygen atoms. Linus Pauling, a pioneer in chemical bonding theory, provided foundational principles applicable to understanding this structure. Specifically, molecular orbital theory helps explain why carbon monoxide deviates from simple octet rule expectations. The representation of carbon monoxide Lewis structure can be visually demonstrated using tools like ChemDraw, illustrating the triple bond and formal charges inherent in this molecule. Further analysis may involve consulting the resources available at institutions like the National Institute of Standards and Technology (NIST) for accurate spectroscopic data related to its bonding characteristics.
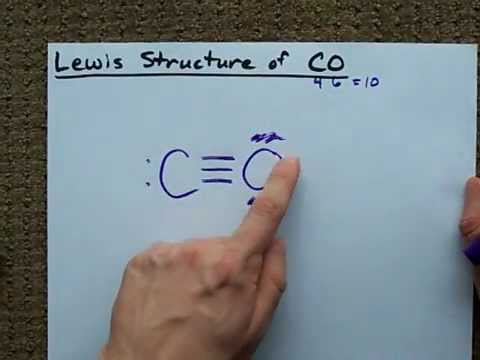
Image taken from the YouTube channel chemistNATE , from the video titled Lewis Structure of CO (Carbon Monoxide) .
Carbon monoxide (CO), a seemingly simple molecule, holds a place of both vital importance and potential danger in the realm of chemistry and everyday life. Composed of a single carbon atom and a single oxygen atom, its chemical formula belies a complex electronic structure that dictates its unique properties and reactivity.
Properties, Uses, and Dangers of Carbon Monoxide
CO is a colorless, odorless, and tasteless gas, making it notoriously difficult to detect without specialized equipment. This characteristic is what makes it so dangerous.
It is produced through the incomplete combustion of carbon-containing materials. Sources range from vehicle exhaust and malfunctioning furnaces to burning wood and charcoal.
The insidious nature of carbon monoxide lies in its ability to bind to hemoglobin in the blood much more strongly than oxygen. This process effectively prevents oxygen transport throughout the body, leading to carbon monoxide poisoning, which can cause severe health issues and even death.
Despite its toxicity, carbon monoxide also has industrial applications. It serves as a crucial building block in the synthesis of various chemicals, including pharmaceuticals, plastics, and fuels. It is also used in metallurgy to refine metals.
The Importance of Understanding CO’s Structure
Understanding the chemical behavior of carbon monoxide necessitates a deep dive into its electronic structure, specifically its Lewis structure.
The Lewis structure provides a visual representation of how valence electrons are arranged within the molecule, showcasing the bonds between atoms and the presence of any lone pairs.
In the case of carbon monoxide, the Lewis structure reveals an unusual triple bond between carbon and oxygen. It also shows a unique distribution of formal charges. These structural features are crucial for explaining CO’s reactivity, its ability to bind to metal atoms, and its role in various chemical reactions.
Thesis Statement
A clear understanding of the Lewis Structure of Carbon Monoxide is essential for comprehending its chemical behavior. This understanding is facilitated by knowledge of valence electrons, the octet rule, formal charge calculations, and the resulting triple bond, which collectively dictate CO’s unique chemical properties.
Foundational Concepts: Essential Knowledge for Lewis Structures
Before we can accurately depict the electronic arrangement within carbon monoxide, it’s crucial to establish a firm understanding of the fundamental principles that govern Lewis structures. These principles include valence electrons, the octet rule, and formal charge. Each plays a critical role in determining the most accurate and stable representation of a molecule’s structure.
Valence Electrons: The Key to Chemical Bonding
Valence electrons are the electrons residing in the outermost electron shell of an atom.
They are the most energetic electrons and are, therefore, the ones primarily involved in chemical bonding.
The number of valence electrons an atom possesses dictates its bonding behavior and the types of chemical bonds it can form.
To determine the number of valence electrons, we look to the element’s position on the periodic table.
Elements in the same group (vertical column) have the same number of valence electrons.
Valence Electrons in Carbon and Oxygen
Carbon (C) is in Group 14 (also known as Group 4A) of the periodic table. This means it has four valence electrons.
These four electrons allow carbon to form up to four covalent bonds.
Oxygen (O), on the other hand, is in Group 16 (or 6A). Consequently, oxygen has six valence electrons.
This electron configuration enables oxygen to form up to two covalent bonds.
Octet Rule: Striving for Stability
The octet rule is a guiding principle in understanding how atoms form bonds.
It states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell containing eight electrons, resembling the electron configuration of a noble gas.
This configuration is associated with increased stability.
The octet rule is particularly useful for elements in the second period (like carbon and oxygen).
Octet Rule Exceptions and Carbon Monoxide
While the octet rule is a valuable guideline, there are exceptions.
Some molecules, at first glance, might appear to violate the octet rule.
Carbon monoxide is one such molecule. Initial attempts to draw its Lewis structure satisfying the octet rule for both carbon and oxygen can seem problematic.
However, the concept of formal charge helps to resolve this apparent contradiction, allowing us to understand the molecule’s stability despite the seeming violation.
Formal Charge: Identifying the Most Stable Structure
Formal charge is a theoretical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity.
It is a tool used to evaluate different possible Lewis structures and to determine which one is the most stable and accurate representation of the molecule.
The formal charge helps to predict the distribution of electrons within the molecule.
The Lewis structure with the smallest formal charges on the atoms is generally considered the most stable and dominant structure.
Formula for Calculating Formal Charge
The formal charge on an atom in a Lewis structure is calculated using the following formula:
Formal Charge = (Valence Electrons) – (Non-bonding Electrons) – (1/2 Bonding Electrons)
Where:
- Valence Electrons: The number of valence electrons of the neutral atom.
- Non-bonding Electrons: The number of electrons not involved in bonding (lone pair electrons).
- Bonding Electrons: The number of electrons shared in bonds with other atoms.
Understanding and applying these foundational concepts – valence electrons, the octet rule, and formal charge – is essential for accurately depicting the Lewis structure of carbon monoxide and understanding its chemical behavior.
The journey through valence electrons, octet rules, and formal charges has equipped us with the foundational knowledge necessary to tackle the Lewis structure of carbon monoxide. Now, we’ll put these principles into action, walking through a detailed, step-by-step construction of the CO Lewis structure. This process will not only solidify your understanding of these core concepts but also highlight the nuances involved in accurately representing molecular structures.
Step-by-Step Construction: Drawing the CO Lewis Structure
Let’s embark on a step-by-step journey to construct the Lewis structure of carbon monoxide, a process that illuminates the interplay of fundamental chemical principles.
Step 1: Calculating Total Valence Electrons
The first step in constructing any Lewis structure is to determine the total number of valence electrons present in the molecule.
We know that carbon (C) has four valence electrons, and oxygen (O) has six.
Therefore, the total number of valence electrons in CO is 4 + 6 = 10.
This number represents the total pool of electrons we have to work with when drawing the Lewis structure.
Step 2: Drawing the Initial Skeletal Structure
Next, we draw the basic skeletal structure of the molecule, connecting the atoms with a single bond.
In the case of CO, this is simply C-O.
This single bond represents a shared pair of electrons, accounting for two of the ten valence electrons we calculated earlier.
Step 3: Distributing Electrons to Satisfy the Octet Rule (Initially)
The octet rule dictates that atoms "want" eight electrons in their valence shell to achieve stability.
Let’s initially try to satisfy the octet rule by distributing the remaining eight electrons as lone pairs around the carbon and oxygen atoms.
We could place six electrons around oxygen (three lone pairs) and two electrons around carbon (one lone pair), resulting in: C: - O:::.
However, this leaves carbon with only four electrons (two from the single bond and two from the lone pair) and oxygen with eight.
This does satisfy the octet rule for oxygen, but it leaves carbon significantly short of its octet. This is not an ideal Lewis structure.
Step 4: Introducing Multiple Bonds
To address carbon’s electron deficiency and minimize formal charges, we need to introduce the concept of multiple bonds.
Multiple bonds involve sharing more than one pair of electrons between two atoms. In the case of CO, we’ll explore the possibility of forming a double or triple bond.
By forming multiple bonds, we can simultaneously increase the number of electrons around both carbon and oxygen, bringing them closer to satisfying the octet rule.
Step 5: Forming the Triple Bond between Carbon and Oxygen
To best satisfy the octet rule for both carbon and oxygen, a triple bond is formed.
This involves sharing three pairs of electrons, leaving one lone pair on each atom.
The Lewis structure now looks like this: C≡O. Each atom has one lone pair.
This arrangement gives both carbon and oxygen eight electrons in their valence shell (six from the triple bond and two from the lone pair).
Understanding Sigma (σ) and Pi (π) Bonds
A triple bond isn’t simply three identical bonds. Instead, it comprises one sigma (σ) bond and two pi (π) bonds.
The sigma bond is a single bond formed by the direct, head-on overlap of atomic orbitals. It’s the first bond formed between two atoms.
Pi bonds, on the other hand, are formed by the sideways overlap of p orbitals. They are weaker than sigma bonds and exist above and below the internuclear axis.
Step 6: Calculating Formal Charges
While the triple bond satisfies the octet rule, we still need to calculate formal charges to determine the most stable Lewis structure.
The formal charge is calculated as: Formal Charge = (Valence Electrons) – (Non-bonding Electrons) – (1/2 * Bonding Electrons)
For Carbon: FC = 4 – 2 – (1/2 6) = -1
For Oxygen: FC = 6 – 2 – (1/2 6) = +1
These formal charges indicate a slight charge separation within the molecule, with carbon carrying a negative charge and oxygen carrying a positive charge.
Step 7: Presenting the Final, Optimized Lewis Structure
The final, optimized Lewis structure of carbon monoxide (CO) is therefore:
⁻C≡O⁺
This structure shows the triple bond between carbon and oxygen, along with the formal charges of -1 on carbon and +1 on oxygen.
While it might seem unusual to have formal charges, this structure best represents the electron distribution and stability of the carbon monoxide molecule, and minimizes charge separation overall.
The journey through valence electrons, octet rules, and formal charges has equipped us with the foundational knowledge necessary to tackle the Lewis structure of carbon monoxide. Now, we’ll put these principles into action, walking through a detailed, step-by-step construction of the CO Lewis structure. This process will not only solidify your understanding of these core concepts but also highlight the nuances involved in accurately representing molecular structures.
Analyzing the Structure: Understanding the Carbon Monoxide Bond
With the Lewis structure of carbon monoxide now in hand, it’s time to dissect its features and understand what they tell us about the molecule’s behavior. The structure isn’t just a static representation; it’s a roadmap to understanding CO’s reactivity, bond strength, and unique characteristics.
Bond Order in Carbon Monoxide
The bond order is a fundamental concept for understanding the stability and strength of a chemical bond. It’s defined as the number of chemical bonds between a pair of atoms. In simpler terms, it indicates how many bonds are holding the atoms together.
For carbon monoxide, the Lewis structure reveals a triple bond between the carbon and oxygen atoms. This means that three pairs of electrons are being shared.
Therefore, the bond order of carbon monoxide is 3. This high bond order indicates a strong and relatively short bond, contributing to the stability of the CO molecule.
The Carbon Monoxide Triple Bond: Sigma and Pi Components
The triple bond in carbon monoxide is not a single entity but rather a combination of different types of covalent bonds. It comprises one sigma (σ) bond and two pi (π) bonds.
The sigma bond is formed by the direct, head-on overlap of atomic orbitals, resulting in electron density concentrated along the internuclear axis. It is the strongest type of covalent bond.
Pi bonds, on the other hand, are formed by the sideways overlap of p orbitals, resulting in electron density above and below the internuclear axis. Pi bonds are generally weaker than sigma bonds.
In CO, the sigma bond provides the initial framework, while the two pi bonds further strengthen and shorten the bond between carbon and oxygen. The combination of these three bonds is what makes the carbon monoxide bond so strong and gives it its characteristic properties.
Formal Charges and Reactivity
While the triple bond provides stability, the formal charges on the carbon and oxygen atoms in CO play a crucial role in determining its reactivity. In the most stable Lewis structure of CO, carbon carries a formal charge of -1, and oxygen carries a formal charge of +1.
This charge separation creates a dipole moment in the molecule.
The negatively charged carbon atom is more likely to act as a nucleophile, meaning it is attracted to positively charged species or electron-deficient centers. Conversely, the positively charged oxygen atom is more likely to act as an electrophile, meaning it is attracted to negatively charged species or electron-rich centers.
This charge distribution influences how carbon monoxide interacts with other molecules and participates in chemical reactions. For example, the carbon atom in CO can coordinate to metal centers in organometallic compounds, a crucial step in many catalytic processes. Understanding the formal charges, therefore, is essential to predicting CO’s behavior in various chemical environments.
FAQs: Understanding Carbon Monoxide Lewis Structure
What exactly does the Lewis structure of carbon monoxide show?
The carbon monoxide lewis structure illustrates how the carbon and oxygen atoms are bonded together. It shows a triple bond between carbon and oxygen, including one coordinate covalent bond, along with lone pairs of electrons on each atom.
Why is there a triple bond in the carbon monoxide Lewis structure?
Carbon and oxygen both need to achieve an octet (8 valence electrons) to be stable. The triple bond, with one bond being coordinate covalent, allows both carbon and oxygen to fulfill this octet rule in the carbon monoxide lewis structure.
Why does carbon monoxide have a coordinate covalent bond?
In the carbon monoxide lewis structure, one of the bonds is a coordinate covalent bond where both electrons in the bond are donated by the oxygen atom. This allows both atoms to achieve a formal charge closer to zero, contributing to the stability of the molecule.
Is the carbon monoxide Lewis structure the only way to represent the molecule?
While the shown carbon monoxide Lewis structure is the most commonly accepted representation, other resonance structures can be drawn. However, the structure showing a triple bond (including a coordinate covalent bond) and formal charges as close to zero as possible best represents the bonding in carbon monoxide.
Alright, hopefully, you’ve now got a solid grasp on the carbon monoxide Lewis structure! Go forth and conquer those chemistry problems. Until next time!