Meso compounds, a fascinating topic in stereochemistry, possess unique characteristics stemming from their internal plane of symmetry. A key question arises when considering these molecules: are meso compounds superimposable on their mirror images? Indeed, the very definition hinges on this property. Understanding this relies on grasping the principles taught within organic chemistry curricula, particularly concerning chirality centers and molecular symmetry. The International Union of Pure and Applied Chemistry (IUPAC) provides guidelines for nomenclature, helping clarify why, despite having chirality centers, these substances don’t exhibit optical activity. Thus, examining how are meso compounds superimposable becomes crucial in analyzing molecular properties.
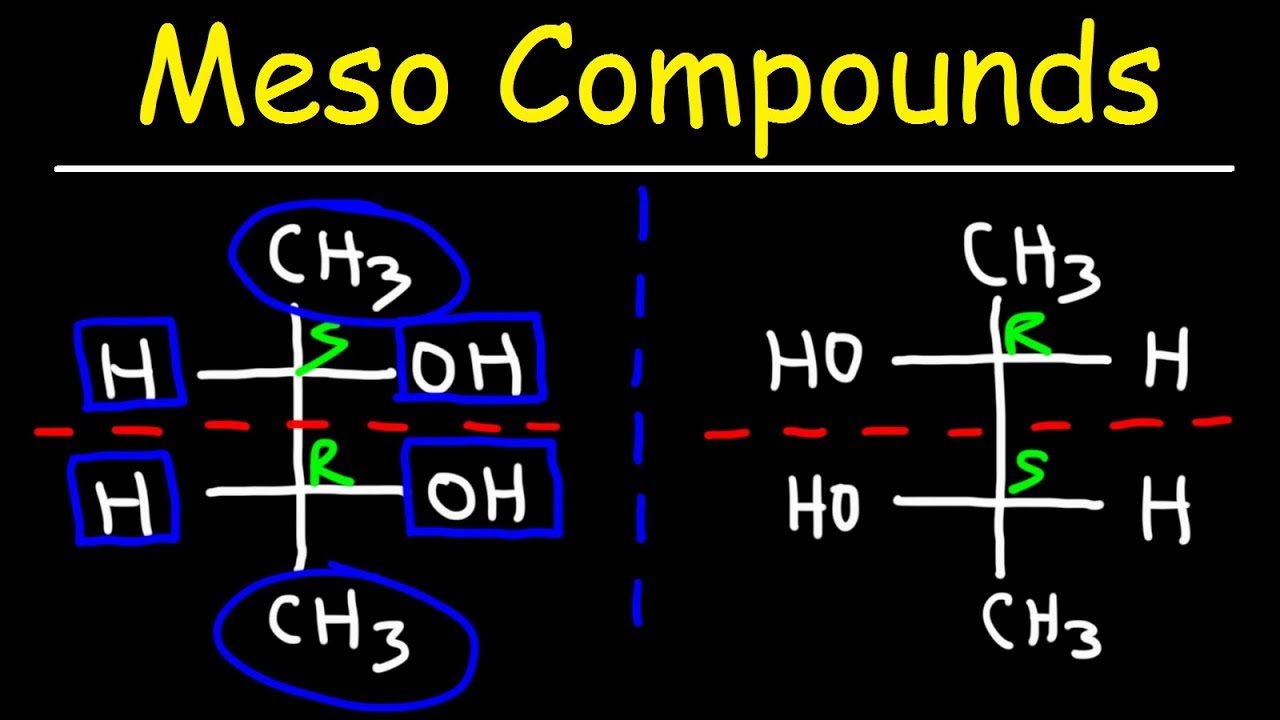
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled Meso Compounds .
Are Meso Compounds Truly Superimposable? Decoding Molecular Symmetry
The core question we need to address is: "are meso compounds superimposable?" To understand this, we need to break down the concept of meso compounds and superimposability, and then examine how these two concepts interact. The ideal article layout should facilitate a clear and logical understanding.
Defining Chirality and Stereoisomers
Before tackling meso compounds, we must first establish the fundamental concept of chirality.
-
Chirality: This refers to the property of a molecule being non-superimposable on its mirror image. Think of your hands – they’re mirror images, but you can’t perfectly overlap them. Such molecules are described as chiral.
-
Stereoisomers: These are molecules with the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
-
Enantiomers: Stereoisomers that are mirror images of each other and are non-superimposable.
-
Diastereomers: Stereoisomers that are not mirror images of each other. Meso compounds fall into this category.
-
Introducing Meso Compounds
Meso compounds represent a special case within stereoisomers. They appear to be chiral (contain stereocenters, which are atoms bonded to four different groups), but, due to internal symmetry, are actually achiral.
The Key Feature: Internal Plane of Symmetry
The presence of an internal plane of symmetry is the defining characteristic of a meso compound.
- This plane divides the molecule into two halves that are mirror images of each other.
- This internal mirror image effectively cancels out any potential chirality.
Identifying Stereocenters
Meso compounds do possess stereocenters. The presence of stereocenters is a prerequisite, but not a guarantee, of chirality. The internal plane of symmetry is what renders the molecule achiral, despite having stereocenters.
- Careful examination of the molecule is crucial to locate stereocenters.
- The focus should then shift to identifying any symmetry elements.
Superimposability Explained
Superimposability refers to whether an object (in this case, a molecule) can be placed on top of its mirror image such that all parts match perfectly. If it can, the object is superimposable and achiral. If it cannot, it is non-superimposable and chiral.
Rotation and Superimposition
To determine superimposability, one needs to mentally (or physically using models) rotate and manipulate the molecule. If, after any rotation, the molecule exactly matches its mirror image, it’s superimposable.
The Challenge with Meso Compounds
The tricky part about meso compounds is that, at first glance, they might seem non-superimposable on their mirror images due to the presence of stereocenters. However, the internal plane of symmetry allows them to be rotated into a conformation that is superimposable.
Visual Examples and Illustrations
A critical component of this explanation is the use of visual aids.
- Diagrams: Show examples of molecules with and without stereocenters.
- 3D Representations: Illustrate the internal plane of symmetry in a meso compound.
- Step-by-Step Animations: Demonstrate the rotation of a meso compound into its superimposable conformation.
For example, tartaric acid can exist as a chiral (enantiomeric) or achiral (meso) form. A table could be used to compare and contrast them:
| Feature | Chiral Tartaric Acid | Meso Tartaric Acid |
|---|---|---|
| Stereocenters | Yes (two) | Yes (two) |
| Symmetry Plane | No | Yes |
| Superimposable | No | Yes |
| Optical Activity | Optically Active | Optically Inactive |
Addressing Common Misconceptions
It’s important to directly address common misunderstandings:
- Stereocenters Always Imply Chirality: This is incorrect. Meso compounds demonstrate that stereocenters, in the presence of an internal plane of symmetry, do not guarantee chirality.
- Meso Compounds are Just Rotated Versions: While rotation is used to demonstrate superimposability, meso compounds aren’t simply rotations of chiral compounds. They possess inherent symmetry from the outset.
- Confusing Meso Compounds with Achiral Compounds: Achiral compounds do not necessarily have stereocenters. Meso compounds do have stereocenters but are achiral due to their symmetry.
Frequently Asked Questions About Meso Compounds
This FAQ section addresses common questions about meso compounds and their unique superimposability properties discussed in the main article. We hope this helps to further clarify your understanding of these fascinating molecules.
What exactly makes a compound "meso"?
A meso compound is a molecule with multiple chiral centers that is nonetheless achiral due to an internal plane of symmetry. The stereochemistry at the chiral centers cancels each other out, resulting in an overall non-chiral molecule.
So, are meso compounds superimposable on their mirror images?
Yes, meso compounds are superimposable on their mirror images. This is the defining characteristic of a meso compound, differentiating it from chiral compounds which are non-superimposable on their mirror images (enantiomers). The internal plane of symmetry allows for this superimposition.
How does the internal plane of symmetry make a molecule achiral?
The internal plane of symmetry effectively creates a "mirror image" within the molecule itself. This means that even though the molecule contains chiral centers (stereocenters), the two halves of the molecule are mirror images of each other, negating the chirality introduced by each stereocenter.
Can a molecule with only one chiral center be a meso compound?
No, a molecule needs at least two chiral centers to potentially be a meso compound. A single chiral center inherently leads to chirality, meaning it will have a non-superimposable mirror image. The internal compensation of stereochemistry in meso compounds requires multiple chiral centers.
So, next time you’re puzzling over a molecular structure, remember that are meso compounds superimposable! Hopefully, this cleared things up a bit. Happy studying!